How to Improve Medical Device Clinical Trials With The Right Partner Ecosystem
Partnerships are a key to success when it comes time for your company’s next clinical trial. In this blog post, we will illustrate how building an effective partnership ecosystem can speed up timelines while optimizing operational activities in a clinical trial while highlighting a recent success story between Essity, Link2Trials, and Castor.
How to integrate partnerships to overcome clinical trial obstacles
Essity, a global hygiene and health company dedicated to improving well-being through its products and services, was given the opportunity to begin an early feasibility study with a prototype of a bladder sensor using ultrasound for physicians and patients to monitor urinary incontinence.
Senior Clinical Trial Manager at Essity, Sandra Tobisch recalled, “The challenge was to recruit healthy subjects, decrease the number of visits, and perform measurements with our own device in-house—being the sponsor and keeping GDPR issues in mind.”
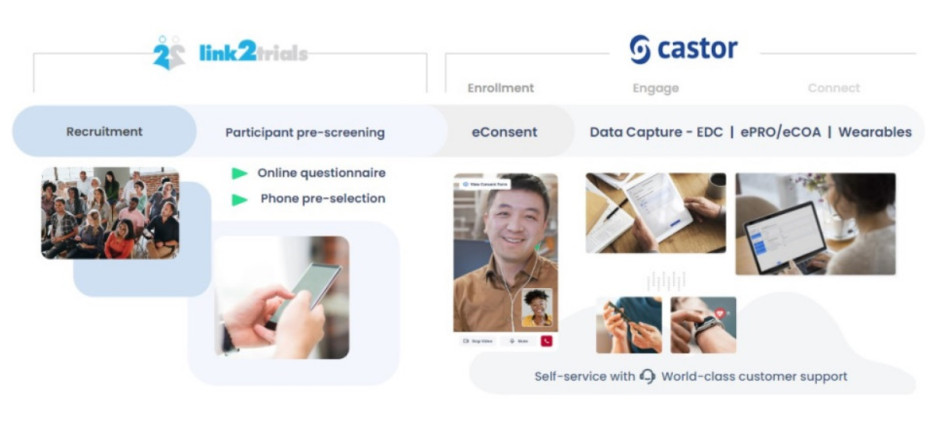
To overcome the trials’ predicted patient recruitment and engagement challenges, Essity turned to Link2Trials, a global patient recruitment company, and Castor, a modular eClinical platform.
Prioritizing interoperability
Interoperability is vital with partnerships for both patients and researchers. Joining multiple data sources and technologies comes with its own set of challenges. This is why picking the right partner can make all the difference in terms of clinical trial success.
One of Essity’s significant concerns was that the patient might not be able to navigate their way through the system and not even make it to the measurement side. The cooperation and teamwork of Castor, Link2Trials, and Essity eased these concerns. From the moment the patient came into contact with the study, it was a seamless process to recruit and enroll patients in the trial.
- Patients signed up with Link2Trials and got a call from their team.
- They were then automatically transferred to the eConsent system with Castor, if screened successfully.
- Castor’s API was able to connect with Link2Trials and enroll patients into the study using eConsent. This automatic connection allowed patients to enroll without having to re-enter data that they’d already entered with Link2Trials.
When speaking to interoperability in the industry now, there is a lot of added complexity. “I think everybody agrees that making trials more patient-centric is a great thing to do, but it often means more complexity,” said Castor’s Derk Arts. “I think we need to consider the fact that there’s not going to be a single vendor that does everything really well. Embracing interoperability will be crucial to transitioning to this modern paradigm where we’ll see more technology components playing together.”
Enabling seamless workflows through standardization
Implementing the right technology is essential when streamlining the patient recruitment and enrollment process.
When integrating technologies, making the process easy on the patients is key. Using an API—like the one Castor used to pull in data from Link2Trials pre-selection—can help streamline the enrollment process to provide a seamless patient experience.
As more APIs begin to tie together, Castor has begun to standardize the process with the goal of rapidly deploying solutions without having to test patient integrations with each clinical trial.
In the Essity trial, getting the patients' data through the system seamlessly was a success. “It was only possible due to high standardization, great cooperation, good monitoring, and proper database setup,” said Tobisch.
How great partnerships can help clinical trial success
In addition to a more streamlined patient experience, medical device, and diagnostic companies can benefit from strategic partnerships like the ones made in the Essity trial, including a 50–65% increase in recruitment rates, 40% higher patient retention—resulting in a 25% cost reduction to develop new products—and a wider range of trial participants for more trial inclusivity and diversity.
“It was really important to find healthy individuals and guide them through the system smoothly.” Tobisch expanded. “[The participants] were really happy about the process and the different systems they used. When they showed up at the site, they provided us with great feedback about how easy it was to both get recruited and enroll in the study.”
To learn more about the clinical trial partnership between Castor, Link2Trials, and Essity, watch the full webinar here.
Like or Share
Choose category
Latest posts
- 04 November, 2025
- Link2Trials Q&A_30
- 15 August, 2025
- Link2Trials Q&A_29
- 18 July, 2025
- Link2Trials Q&A_28
- 04 July, 2025
- Link2Trials Q&A_27
- 03 June, 2025
- Link2Trials Q&A_26

 Argentina
Argentina Australia
Australia Balgarija
Balgarija België
België Canada
Canada Česko
Česko Chile
Chile China (中国)
China (中国) Colombia
Colombia Danmark
Danmark Deutschland
Deutschland England
England España
España France
France Ireland
Ireland Italiana
Italiana Lietuva
Lietuva Magyarország
Magyarország Nederland
Nederland New Zealand
New Zealand Österreich
Österreich Polska
Polska Schweiz
Schweiz Singapore
Singapore Slovenija
Slovenija Slovensko
Slovensko Suomi
Suomi Sverige
Sverige United States
United States Israel
Israel


