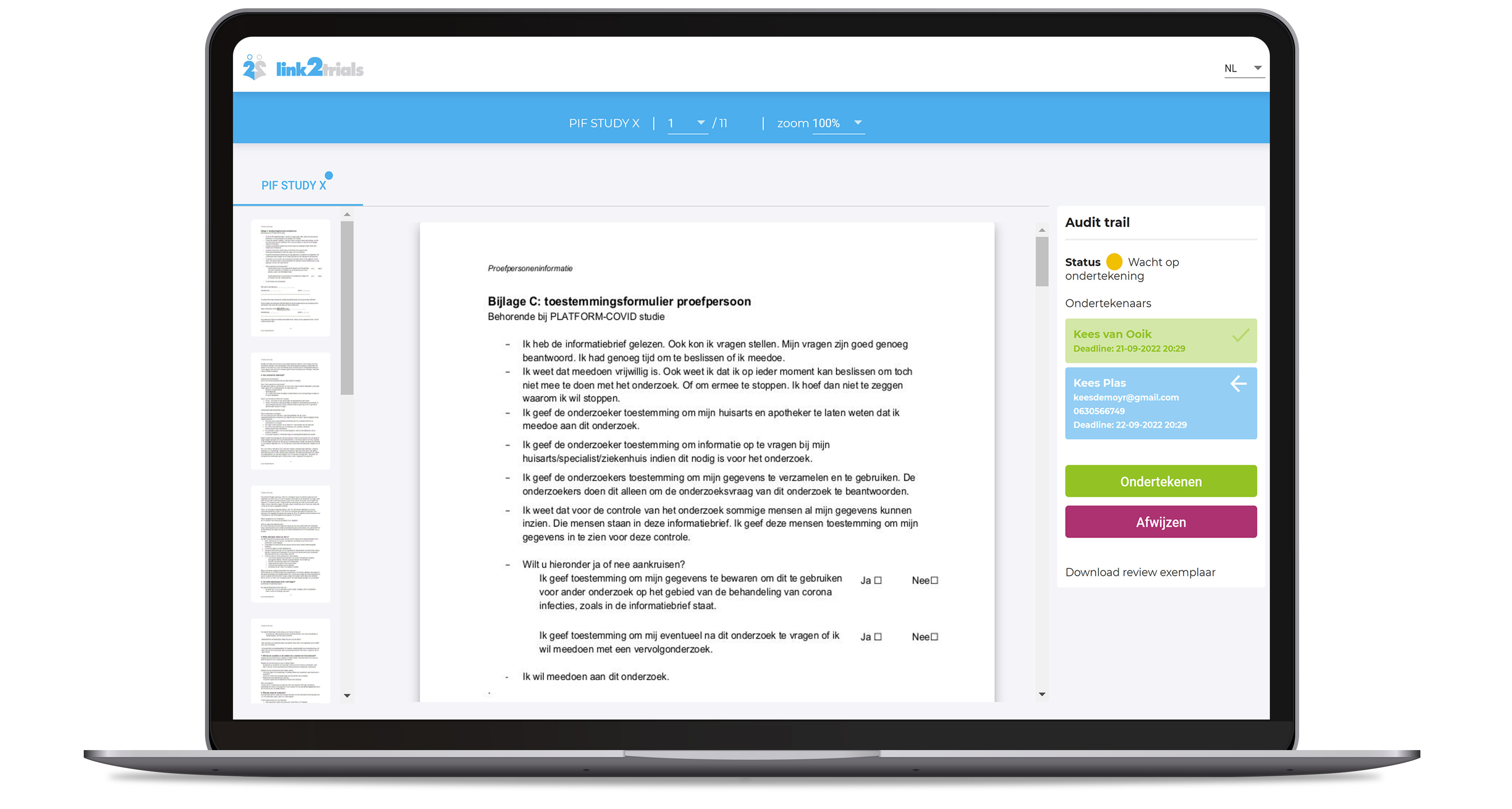
e-Consent; making the patiënt experience better!
Our e-consent module will increase the patient experience by offering both the patient and the site an easy-to-schedule e-consent video meeting including a validated digital signature process. This will not only increase efficiency for both DCT study setups and conventional study setups, but will also reduce the burden for your study teams.
The e-consent module supports videos, images, and other interactive media to increase the patient’s understanding of the study details. It can be integrated in our site workflow management suite for even better support.
In 2018 one of our videos won the European Site Patient Recruitment Innovation Award (SPRIA EU).
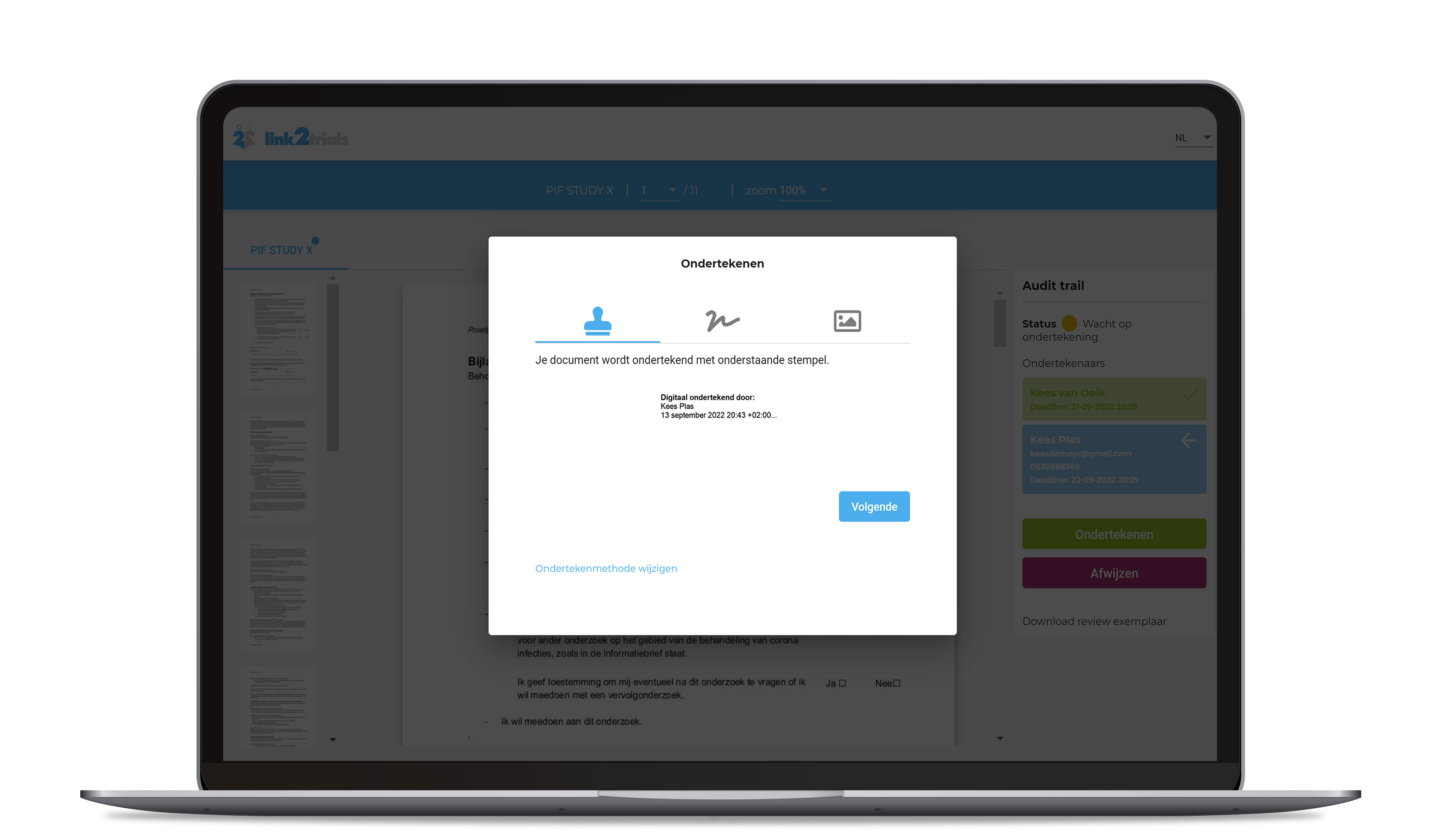
With the eConsent module, a potential study participant can be requested to sign a consent form with advanced electronic signing.
- The signature is uniquely linked to the signatory
- The signature makes it possible to identify the signatory
- The signature is connected to the connected document so that any subsequent changes to the data can be traced.
- The document signed with an electronic signature will be equipped with a “Detached Certificate".
Benefits for your clinical study
- Enhanced patients' understanding of your clinical study
- Different interactive methods for transferring study information
- Lessens the burden on your sites
- Improved patient experience
- A seamless experience for patients
- Real-time process insights for sites

 Argentina
Argentina Australia
Australia Balgarija
Balgarija België
België Canada
Canada Česko
Česko Chile
Chile China (中国)
China (中国) Colombia
Colombia Danmark
Danmark Deutschland
Deutschland England
England España
España France
France Ireland
Ireland Italiana
Italiana Lietuva
Lietuva Magyarország
Magyarország Nederland
Nederland New Zealand
New Zealand Österreich
Österreich Polska
Polska Schweiz
Schweiz Singapore
Singapore Slovenija
Slovenija Slovensko
Slovensko Suomi
Suomi Sverige
Sverige United States
United States Israel
Israel


